Lupine Publishers Group
Lupine Publishers
Menu
ISSN: 2637-4609
Research Article(ISSN: 2637-4609) 
Crystal and Molecular Docking Studies of 3-[Bis-(2- Hydroxy-4,4-Dimethyl-6-Oxo-Cyclohex-1-Enyl)-Methyl]Benzonitrile with Focal Adhesion Kinase Inhibitors Volume 1 - Issue 3
K S Kiran1,2*, M K Kokila1, Guruprasad R3 and Prashantha K4
- 1Department of physics, Bangalore University, India
- 2Department of physics, School of Engineering and Technology, Jain University, India
- 3Scientific Director, Durga Femto Technologies & Research, India
- 4Department of Biotechnology, PES University, India
Received: January 25, 2018; Published: February 13, 2018
Corresponding author: K S Kiran, Department of physics, Bangalore University, Bangalore, Karnataka, India
DOI: 10.32474/AOICS.2018.01.000114
Abstract
Cyclohexane is a non planar molecule and the shape of which is vaguely resembles a chair. The conformation of cyclohexane molecule is constantly changing with the atom on the left which is currently pointing down flipping up and the one on the right flipping down. During the process, another (slightly less stable) form of cyclohexane is formed known as the "boat" form. In this arrangement, both of these atoms are either pointing up or down at the same time. It is a cyclic alkane that melts at 6 °C and boils at 81 °C. It is nearly insoluble in water.
Introduction
Cyclohexane is found naturally to some extent in petroleum but is prepared commercially by catalytic hydrogenation of benzene.It is widely used as a solvent and in making certain compounds used in the preparation of nylon. The biological importance of cyclohexane has resulted in the synthesis of substituted derivatives which show potent pharmaceutical activities like antimicrobial, ant diabetic, anticancer antipsychotic, anesthetic, expectorant activities [1]. Focal adhesion kinase is a cytoplasmic non-receptor protein tyrosine kinase which was isolated for the first time by co- immunoprecipitation of tyrosine-phosphorylated proteins from cells transformed with Rous sarcoma virus v-Src. Non-receptor protein-tyrosine kinase (PTK2/FAK1) that plays an essential role in regulating cell migration, adhesion, spreading, reorganization of the actin cytoskeleton, formation and disassembly of focal adhesions and cell protrusions, cell cycle progression, cell proliferation and apoptosis. It is required for early embryonic and placenta development. It also required for embryonic angiogenesis, normal cardiomyocyte migration and proliferation, and normal heart development. It regulates axon growth and neuronal cell migration, axon branching and synapse formation; required for normal development of the nervous system. It plays a role in osteogenesis and differentiation of osteoblasts. It helps in function of integrin signal transduction, but also in signaling downstream of numerous growth factor receptors.
Material and Methods
For crystallographic analysis data collection and cell refinement was done using CAD-4, Data reduction using MOLEN, Structure solution and refinement using SHELX97, Molecular graphics using ORTEP & PLATON, Material for publication using SHELX97 and for protein-ligand interaction using Autodock software. File format conversion of the coordinates using openbabel.
Crystalization of Kch2
The crystals of the compound 3-hydroxy-2-((2-hydroxy-4, 4- dimethyl-6-oxocyclohex-1-enyl) (4-methoxyphenyl) methyl)-5,5- dimethylcyclohex-2-enone was grown by slow evaporation technique using ethanol as solvent. The X-ray intensity data of the crystals were collected on a Bruker smart CCD diffract meter on graphite monochromatic Moka radiation.
Protein ligand interaction
The docking analysis of the Crystal Structure of Focal Adhesion Kinase Domain Complexed with 7H-Pyrrolo [2,3-d] pyrimidine Derivative (PDB:2ETM) was carried using Auto dock 4.2. The reference ligand 7PY (7-Pyridin-2-Yl-N-(3,4,5-Trimethoxyphenyl)- 7h-Pyrrolo [2,3-D]pyrimidin-2-Amine) was selected for binding site analysis and respective amino acids are considered for gridbox construction. The docking site for KCH2 on 2ETM was defined at the position of the co-crystallized ligand 7PY by using PyRX 0.8 interface with grid box size of 56 x 55 x 56, spacing of 0.375, grid centre -1.220, 11.661 and 6.401. From the estimated free energy of ligand binding (AG), the inhibition constant (Ki) for each ligand was calculated Docking of the protein ligand complex was mainly targeted to the predicted active site. The interaction was carried out to find the favorable binding geometries of the ligand with the protein. The selected residues of the receptor were defined to be part to the binding site. The molecules binding to a receptor, ideally must inhibit its function, and thus act as drug. The collection of bis cyclohexyl diols derivatives and receptor complexes were identified via docking. The scoring functions with their parameters were read from the score obtained. The entire series of ligands in the data set was docked into the active site of FAK protein, using the same protocol. The docking poses were saved for the ligand and were ranked according to their score function. The pose possessing the highest dock score was selected for further analysis. PyMOL and Ligplot+ were used for docking conformation. Only the best pose (the one with the lowest binding energy) was considered as a potential ligand.
Results and Discussion
The molecule, C24H27NO4 crystallizes in monoclinic space group P21/c with unit cell dimensions, a=11.8048(13) Å, b=11.46769(13) Å, c=16.52539(19) Å and Z=4. The ORTEP diagram of the ligand showing 50% probability displacement ellipsoids is shown in Figure 1. The crystal data of the compound are shown in Table 1. The bond lengths and bond angles are generally within normal range. The cyclohexenone ring A (C6/C5/C4/C1/C2/C3) and the benzene ring B (C22-C27) are not planar. The cyclohexene ring constitutes a part of the tetra benzonitrile fused ring. The bond length O8-C13 is 1.26Å and C1-C2 is 1.38Å for the both cyclohexene ring. A part of the benzonitrile fused ring system has a flattened halfchair conformation that approximates an envelope conformation (in which the methylene C atom bearing the dimethyl substituent represents the flap) as five of the six atoms lie on a plane [2,3]. The mean plane of the cyclohexene ring with the hydroxyl substituent is approximately perpendicular to the mean plane of the tetra benzonitrile system. Adjacent molecules are linked by an O-H...O hydrogen bond to form a chain running along the b-axis of the monoclinic unit cell. The dihedral angle between the benzonitrile ring and cyclohexene ring is 70.8°. Relatively strong intermolecular O-H...O hydrogen bonds are observed. In the crystal, molecules are linked by C-H...O hydrogen bonds, forming a chain along the c-axis direction. There is an intermolecular hydroxy-ketone O-H...O interaction between the two substituted cyclohexane rings as well as a short intermolecular phenol-methoxy O-H...O interaction. The packing diagram C-H...O and O-H...O interactions is shown in Figure 2 [4].
Table 1: Docking interaction details of KCH2 and 7PY against FAK protein.
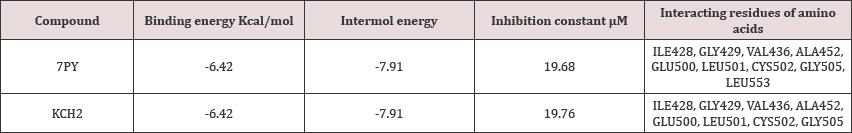
Figure 1: Ortep diagram of 3-hydroxy-2-((2-hydroxy-4, 4-dimethyl-6-oxocyclohex-1-enyl) (4-methoxyphenyl) methyl) - 5, 5-dimethylcyclohex-2-enone.
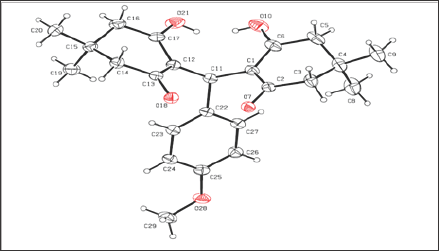
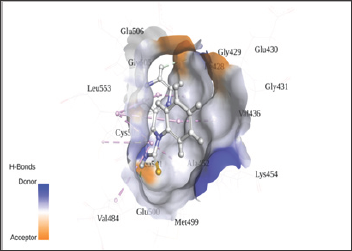
The in silico interaction of 7-pyridin-2-yl-n-(3,4,5-trimeth- oxyphenyl)-7h-pyrrolo[2,3-d]pyrimidin-2-amine (7PY) to 2ETM showed the least binding energy of -6.42kcal/mol. From the PDB sum it is observed that 7PY forms two hydrogen bonds with CYS502 with bond length of 3.11Å and 2.91Å. The conformation with least binding energy and most stability based on cluster analysis was taken for docking analysis. In the Autodock predicted interaction, it shows the interaction as hydrophobic and due to this the inhibition constant value increased from 0.212μM to 19.68 μM. Since Autodock calculates the inhibition constant from the binding energy and both 7PY and KCH1 has same energy, we observe nearly the same IC50 values and may act by competitive inhibition. The interacting residues in both 7PY and KCH2 are almost conserved. KCH1 shows a hydrophobic interaction as showed in Figure 2 and Figure 3. The docking interaction details are represented in Table 1. Since the synthesized chemical compound showed good fit with the protein, the bioactive compound may be used as a potent inhibitor to block the action of FAK protein [5].
Figure 2: Interaction of KCH2 with 2ETM.
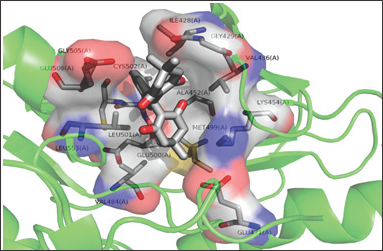
Figure 3: Interaction of KCH2 in the active site of FAK protein.
Conclusion
In the present study topological analysis of3-hydroxy-2-((2- hydroxy-4, 4-dimethyl-6-oxocyclohex-1-enyl) (4-methoxyphenyl) methyl) - 5, 5-dimethylcyclohex-2-enone is performed using crystallographic method. The results were extrapolated to molecular docking analysis to investigate the structure-activity relationship between the inhibitory features of KCH2 ligand. From the Auto Dock result it can be concluded that the bioactive compound KCH2 can be used as a potent inhibitor to block the action of FAK protein when it is over expressed. Further, the bioactive compound will be analyzed by molecular dynamics studies and then in vivo studies for detailed investigations. Further co-crystallization of protein- ligand complex and animal trials followed by biopharmaceutical scale up feasibilities could be encouraged [6].
Acknowledgement
The authors are thankful to CCD facility, IISC, Bangalore.Authors thanks to Director, School of Engineering and technology,Jain University for the support and encouragement
References
- Wilson, Gisvold (1998) Text book of Organic and Medicinal and Pharmaceutical Chemistry, 10th (edn), New York, Lippincott-Raven Publishers 342-393.
- Sughanya V, N Sureshbabu (2012) 2,2'-[(4-Ethoxyphenyl) methylene] bis (3-hydroxy-5,5-dimethylcyclohex-2-en-1-one), Acta Cryst 68(9): pp2638.
- Xiao-Hui Yang, Yong-Hong Zhou, Meng Zhang, Li-Hong (2011) Hu3- Hydroxy-2-[(4-hydroxy-3,5-dimethoxyphenyl)(2-hydroxy-4,4- dimethyl-6-oxocyclohex-1-en-1-yl)methyl]-5,5-dimethylcyclohex-2-en- 1-one. Acta Cryst E67: pp492.
- Sureshbabu N, Sughanya (2013) 6-Hydroxy-5-[(2-hydroxy-4,4- dimethyl-6-oxocyclohex-1-enyl) (4-nitrophenyl)-methyl]-1,3-dimethylpyrimidine-2,4(1H,3H)-dione. Acta. Cryst 69: 1690-1691.
- Wang J, Zu J, Xu G, Zhao W, Jinglong Y (2014) Inhibition of focal adhesion kinase induces apoptosis in human osteosarcoma SAOS-2 cells. Tumour Biol 35: 1551-1556.
- Wang R, Lu Y, Wang S (2003) Comparative evaluation of 11 scoring functions for molecular docking. J Med Ch 46: 2287-2303.

Top Editors
-

Mark E Smith
Bio chemistry
University of Texas Medical Branch, USA -

Lawrence A Presley
Department of Criminal Justice
Liberty University, USA -

Thomas W Miller
Department of Psychiatry
University of Kentucky, USA -

Gjumrakch Aliev
Department of Medicine
Gally International Biomedical Research & Consulting LLC, USA -

Christopher Bryant
Department of Urbanisation and Agricultural
Montreal university, USA -

Robert William Frare
Oral & Maxillofacial Pathology
New York University, USA -

Rudolph Modesto Navari
Gastroenterology and Hepatology
University of Alabama, UK -

Andrew Hague
Department of Medicine
Universities of Bradford, UK -

George Gregory Buttigieg
Maltese College of Obstetrics and Gynaecology, Europe -

Chen-Hsiung Yeh
Oncology
Circulogene Theranostics, England -
.png)
Emilio Bucio-Carrillo
Radiation Chemistry
National University of Mexico, USA -
.jpg)
Casey J Grenier
Analytical Chemistry
Wentworth Institute of Technology, USA -
Hany Atalah
Minimally Invasive Surgery
Mercer University school of Medicine, USA -

Abu-Hussein Muhamad
Pediatric Dentistry
University of Athens , Greece

The annual scholar awards from Lupine Publishers honor a selected number Read More...